
This lab investigates a typical mixture problem and the effect of different
initial conditions and various run times on the solution of the governing ODE.
The ODE here is based on: rate of change = in - out. i.e.

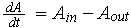
Problem: A rock contains two radioactive isotopes

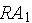 and
and

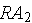 that belong to the same radioactive series; that is
that belong to the same radioactive series; that is

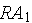 decays into
decays into

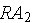 which then decays into stable atoms. Assume that the rate that
which then decays into stable atoms. Assume that the rate that

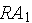 decays into
decays into

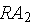 is
is

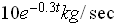 and that the rate of decay of
and that the rate of decay of

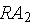 is proportional to the mass
is proportional to the mass

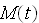 of
of

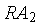 present. If the decay constant is
present. If the decay constant is

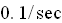 using the time periods and initial conditions given below create the graphs of
the particular solutions as indicated using ODE Architect and print each of
the groups 1-4 below (1 print out for each group).
using the time periods and initial conditions given below create the graphs of
the particular solutions as indicated using ODE Architect and print each of
the groups 1-4 below (1 print out for each group).
1. For a time lapse of

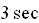 on the same grid graph the particular solutions for :
on the same grid graph the particular solutions for :
Initial conditions:

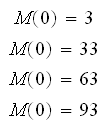
2. Repeat 1. for 13 sec.
3. Repeat 1. for 23 sec.
4. Repeat 1. for 53 sec. print this graph with the slope field.
5. Now write out a discussion (one page max.) about your observations of what seems to be happening. Include observations as to how the projection changes as the time period increases and how the various initial conditions effect these changes.
This document created by Scientific Notebook 4.1.