PHYS 1407 Ė Conceptual Physics II
Photoelectric Effect II - The Einstein Equation
Leader: _________________________††††††††† Recorder: __________________________
Skeptic: _________________________†††††††† Encourager: ________________________
Materials
Planckís Constant Apparatus
Mercury Lamp
2 x DMMís with red and black banana cables instead of leads
Small Lab Jack
Safety
1.† The mercury lamps used in this experiment emit significant amounts of UV radiation.† Do not look directly into them.
Introduction
††††† In the photon interpretation of light, light consist of small pockets of energy called photons.† Each photon carries an amount of energy that is proportional to the frequency of the light.† The energy contained in a single photon is given by
Ephoton = planckís constant x frequency.† Planckís constant is a very small number which sets the scale for which quantum mechanical effects are important.† Itís value is
6.63 x 10-34 Js.
Q1.† If light is shined on a metal where the energy of a single photon is less than the energy that binds an electron to a metal, will photoelectrons be emitted?
Q2.† If light is shined on a metal where the energy of a single photon is greater than the energy that binds an electron to a metal, will photoelectrons be emitted?
Q3.† What determines the threshold frequency for when light shining on a metal will result in photoelectrons being emitted?
Q4.† If a photon has energy than the bonding energy of the electron, what happens to the extra energy when the electron is emitted?
In his miraculous year of 1905, Einstein wrote a paper interpreting the photoelectric effect in terms of photons.† It was for this paper that Einstein eventually won the Nobel Prize in Physics in 1921.† A key idea of this paper was applying conservation of energy to the photoelectric effect, producing what is called the Einstein equation.† The Einstein equation relates the energy of the photon, the binding energy of the electron to the metal, and maximum kinetic energy of the electron once it is emitted.
Q5.† Write an expression for conservation of energy for the photoelectric effect by filling in the blanks below using Ephoton, Ebinding, KEmax
________ = _______ + ________
Procedure
1.† Set-up the DMMís
††††† On one of the DMMís if not already there, plug the red banana cable into the 200 ma socket and the black banana cable into the COM socket.† This DMM will be referred to as the ammeter for the remainder of this activity.† Plug the other end of the red banana cable into the socket labeled Ammeter + and the black banana cable into the socket labeled Ammeter - on the Planck Apparatus.†
††††† On the other DMM plug the red cable into the socket labeled V-Ω and the black cable into the socket labeled COM.† This DMM will be referred to as the voltmeter for the remainder of this activity.† Plug the other end of the red cable into the socket labeled †Voltmeter + and the black cable into the socket labeled Voltmeter Ė on the Planckís Apparatus.
††††† Have the instructor verify that you have set up the DMMís correctly.
2.† Set-up the Light Source
††††† Place
the mercury discharge lamp on the lab jack.†
Adjust the height of the jack so that the lamp is at the height of the
window on the side of the Planck Apparatus.†
Donít plug the lamp in yet.
3.† Zero the Current
Turn on the ammeter to the 200 μA
DC setting.† Turn on the voltmeter to the
20
4.† Measure the Stopping Potential
Plug in the mercury lamp.† It will take a few seconds for it to warm up.† The window through which the light enters is covered by filters that only allow certain colors of light to pass.† The filters are chosen to coincide with strong emission lines from mercury.† The four wavelengths chosen are 390 nm, 430 nm, 540 nm, and 580 nm.
Q6.† Rank the energy per photon for the light transmitted by each of the four filters from least to greatest.
Least Energy 1. _____ 2. _____ 3. ______ 4.† ________ Most Energy
Q7.† Explain your ranking.
Rotate the filters so that UV-39 (390 nm) filter lies in front of the window.
Q8.† Record the photocurrent detected.
Slowly increase the photocell voltage to its maximum value.
Q9.† What happens to the photocurrent as you turn up the photocell voltage?
D10.† Carefully find the voltage, as read by the voltmeter, where the photocurrent first becomes zero as you turn up the photocell voltage.† Record this potential in the table below. This voltage is called the stopping potential.
|
Wavelength (nm) |
Stopping Potential (V) |
|
|
|
|
|
|
|
|
|
|
|
|
Q11.† What is the sign of the charge of an electron?
Q12.† Will an electron be attracted to a positively charged electrode or a negatively charged electrode?
The figure below shows what happens as you turn up the photocell voltage.† As you turn up the photocathode voltage, the electrode opposite the photocathode, the anode, becomes more negatively charged.
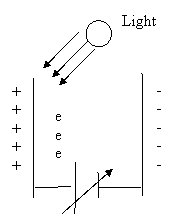
Q13.† Will electrons be attracted to or repelled by the anode?
Q14.† If the electrons are repelled by the anode, then why do you measure any photocurrent in the circuit at all?
P15.† Will the value of the stopping potential you measure be greater, less or the same when you use 430 nm light?
P16.† Will the value of the stopping potential you measure be greater, less or the same when you use 540 nm light?
P17.† Will the value of the stopping potential you measure be greater, less or the same when you use 580 nm light?
D18.† Measure the stopping potential for the other filters.† If no photocurrent is detected for a filter (sometimes happens with the 580 nm light) write no current in the table.
Q19.† Were your predictions correct?† If they werenít, explain how to correct them.
Q20.† What type of relationship is there between the stopping potential and the maximum kinetic energy of the photoelectrons?
S21.† Describe in your own words what happens to the energy in the photoelectric effect starting with the incoming photon.